Perfect tandem: why Ruangan is effective against the background of virus suppression
In modern hepatology, the treatment of chronic hepatitis B (CHB) has achieved
Ruangan is a drug developed on the basis of university clinics in China and used for fibrosis and cirrhosis of the liver of any etiology.

✔ Analysis improvement begins
✔ Reduces inflammation in the liver, gastrointestinal tract, and abdominal organs
✔ Regeneration begins in the liver tissues
✔ Stops the development of fibrosis, cirrhosis
✔ Weakness and depression go away. The risk of liver cancer is reduced by 50%
✔ Liver and kidneys are restored, rollback to 1-1. 5 degrees of fibrosis
✔ Fibrosis 3 rollback to F1-2, cirrhosis rollback to F2-3
✔Platelet growth and normalization of ALT, AST, and AFP occur
✔ Further protective liver protection is valid for 2 years
 Improvement in analysis begins
Improvement in analysis begins  Reduces inflammation in the liver, gastrointestinal tract, abdominal organs
Reduces inflammation in the liver, gastrointestinal tract, abdominal organs
 Regeneration begins in the liver tissues
Regeneration begins in the liver tissues  Stops the development of fibrosis, cirrhosis
Stops the development of fibrosis, cirrhosis
 Weakness and depression go away. The risk of liver cancer is reduced by 50%
Weakness and depression go away. The risk of liver cancer is reduced by 50%  Liver and kidneys are restored, rollback to 1-1. 5 degrees of fibrosis
Liver and kidneys are restored, rollback to 1-1. 5 degrees of fibrosis
 Liver and kidneys are restored, rollback to 1-1. 5 degrees of fibrosis
Liver and kidneys are restored, rollback to 1-1. 5 degrees of fibrosis  Liver and kidneys are restored, rollback to 1-1. 5 degrees of fibrosis
Liver and kidneys are restored, rollback to 1-1. 5 degrees of fibrosis 11 components extracted and enhanced by the synthesis of molecules based on components of plant and animal origin, including:
Biejia
Huangqi
Chishao
Sanqi
Ezhu
Cordyceps
Placenta
and other components included in the preparation.
Fibrosis and cirrhosis of the liver, including:
— alcoholic origin
-toxic nature
— autoimmune origin
-compensated cirrhosis of the liver
Chronic viral hepatitis B and D (delta hepatitis)
– as part of complex antiviral therapy
for fatty liver disease (steatosis, steatohepatitis)
Conditions after a history of viral hepatitis C
— as part of maintenance therapy to reduce the risk of progression of fibrotic changes
Liver damage associated with chemotherapy
— during and after chemotherapy, as part of maintenance therapy
Reduction of cancer risk factors associated with the progression of liver fibrosis.
Custom-made by a pharmaceutical company in China for our patients.
1999.
A Chinese biomedical company active in the production and sale of pharmaceutical products, development of diagnostic medical equipment, and solutions for the diagnosis and management of chronic liver diseases.
The company is listed on the Shenzhen Stock Exchange (ticker 300049).
No. 103 Jiefang Road, Jining District, Ulanqab City, Inner Mongolia Autonomous Region, Китай.
Promote the improvement of healthcare through innovation, high-quality products and the development of medical solutions aimed at treating liver diseases and improving the quality of life of patients.
Development, approval and market launch of Fufang Biejia Ruangan Tablets — one of the earliest anti-fibrotic drugs in China for the treatment of fibrosis and related liver diseases, as well as business expansion in diagnostic medical technologies and solutions for integrated liver disease management.
Inner Mongolia Furui Medical Science Co., Ltd. carries out research and practical activities in the field of development and production of medical products for the diagnosis and treatment of liver diseases. The company combines drug development, scientific research in the field of liver fibrosis and the creation of diagnostic solutions, including technologies for assessing the state of the liver.





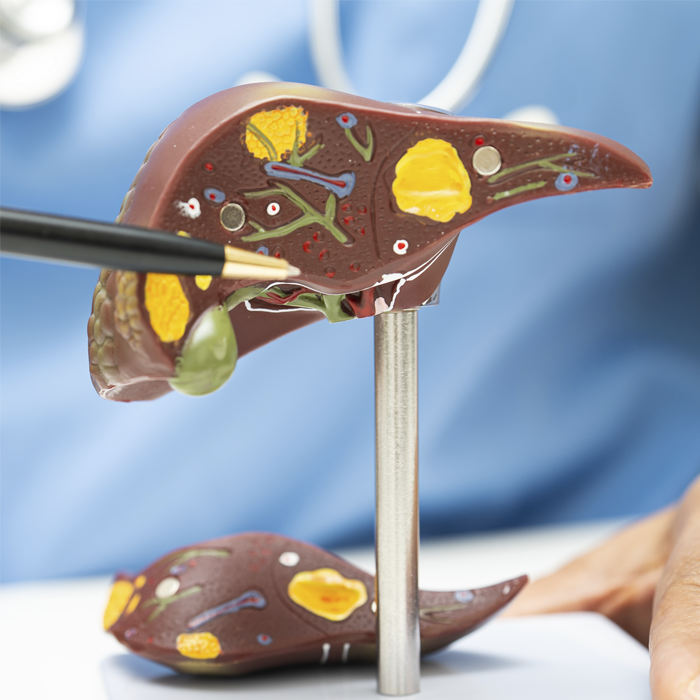
In chronic diseases, it acts precisely on the fibrotic mechanism, restraining and reversing the changes underlying the deterioration of the liver.
Against the background of taking Ruangan, there is a decrease in fibrosis indices and a positive dynamics of the liver structure. Laboratory parameters are returning to normal.
With fibrosis F1, F2, F3 and steatosis 1, 2 degrees-a course of 6 months.
With F4, cirrhosis, steatosis of the 3rd degree-a course of 12 months.
In chronic diseases, it acts precisely on the fibrotic mechanism, restraining and reversing the changes underlying the deterioration of the liver.
Against the background of taking Ruangan, there is a decrease in fibrosis indices and a positive dynamics of the liver structure. Laboratory parameters are returning to normal.
For viral hepatitis, it is used as part of a comprehensive treatment. In case of toxic and alcoholic liver damage, it can be used as the main antifibrotic agent.
Reduces hepatocyte damage, reduces chronic inflammation and improves microcirculation in liver tissue, which is especially important for viral, toxic and alcoholic lesions.
Stops the formation of scars. It starts the recovery of liver cells. Reduces inflammation and reduces the risk of cancer by 2.5 times.*
*Based on data from clinical trials
With fibrosis F1, F2, F3 and steatosis 1, 2 degrees-a course of 6 months.
With F4, cirrhosis, steatosis of the 3rd degree-a course of 12 months.
Ruangan is a combination drug used in the treatment of chronic liver diseases, including fibrosis, fatty hepatosis, chronic hepatitis and cirrhosis.
The drug is used for:
liver fibrosis of various stages
fatty hepatosis
chronic hepatitis
cirrhosis of the liver
as part of complex therapy under the supervision of a doctor.
Ruangan refers to long-term therapy drugs.
The effect is estimated not by days, but by the dynamics of laboratory and instrumental indicators with a course of several months or more.
Evaluation of effectiveness is carried out by blood tests (ALT, AST) and ultrasound or elastometry data during the course, usually after several months of regular use.
Minimum course duration:
from 6 months — with fibrosis of the initial stages and fatty hepatosis
to 48 weeks or more – with severe fibrosis and cirrhosis
, the duration of the course is determined by the doctor based on the dynamics of the liver condition.
The decision to complete or continue therapy is made by the doctor based on objective indicators of the liver condition.
The product is new and most of the materials are in English/Chinese.
Sanctions restrictions — The Ministry of Health of the Russian Federation has not yet released guidelines.
In this regard, the drug is known in the scientific community, but is little known in polyclinics.
Use one of the contacts on the site to contact an official representative.
Enter your full name, address, ZIP code and phone number, diagnosis, and the required number of packages.
Ruangan is taken orally, after meals, as directed by your doctor.
The standard admission schedule specified in the instructions assumes regular admission for a long course (from several months).
Tablets do not require complex schemes:
there is no need to titrate the dose by day,
no special preparation is required,
the reception is easy to integrate into the daily diet.
With a long course, it is important to observe the regularity of admission and undergo liver monitoring on the recommendation of a doctor.
Yes, Ruangan is used as part of complex therapy.
Compatibility with other drugs is determined by the attending physician.
In most cases, yes. However, you need to consult a doctor before using it, which you can get from us.
Fibrosis and chronic liver changes are long-term processes, so therapy is aimed at gradually stabilizing and slowing down pathological changes, and not at a rapid symptomatic effect.
Practically none, as the liver will begin to actively recover (regeneration). The weakness increases, but after 30 days the strength will return, which was not for a long time.
The price is calculated by the manager depending on the duration of your course.
You can read the reviews at the links below:
https://otzyv.pro/reviews/otzyvy-ruangan-508891.html
https://www.yell.ru/moscow/com/kompaniya-glavnoe-zdorove-na-ulice-gorbunova_11970506/reviews/
Yes. Official contract + personal video call with the head of the company A. S. Tkachenko.
Yes. Here are the main sources:
PubMed-RCT on 240 patients with HBV + RG: https://pubmed. ncbi. nlm. nih. gov / 36940891
Frontiers in Pharmacology-Mechanisms of action of Ruangan Granule (network analysis): https://www.frontiersin.org/articles/10.3389/fphar.2021.754807/full
HansPub-Mechanism of action on signaling pathways (TGF-β, PI3K, MAPK, HSC): https://www.hanspub.org/journal/paperinformation.aspx?paperid=97325
WJGnet-Confirmation of clinical efficacy in HBV cirrhosis: https://www.wjgnet.com/1009-3079/full/v29/i4/159.htm

In modern hepatology, the treatment of chronic hepatitis B (CHB) has achieved

When patients are diagnosed with fibrosis or cirrhosis, the prescriptions almost always
In the era of evidence-based medicine (EBM), it is no longer enough
Оставьте свои контакты и мы свяжемся с вами в ближайшее время

